
The Rosenthal–Trotter MS Chair in Neuroimmunology at Washington University School of Medicine in St. Louis details why the COViMS registry can serve as an important instrument for clinicians who treat patients with multiple sclerosis.

The Rosenthal–Trotter MS Chair in Neuroimmunology at Washington University School of Medicine in St. Louis details why the COViMS registry can serve as an important instrument for clinicians who treat patients with multiple sclerosis.

The neurologist at the Barrow Neurological Institute detailed how she has gotten patients to buy into the adjustment to a virtual medical care model, and what challenges she's had to overcome.




These data on the preventive migraine treatment confirmed findings from previous studies, with eptinezumab not only reducing total migraine days, but elongating the duration of consecutive migraine-free days.
The director of the Sleep Disorders Research Program at Cleveland Clinic Lerner College of Medicine discussed new technologies being used to diagnose and treat sleep apnea.

Treatment with CBD resulted in a significant number of patients with TSC reporting being “much” or “very much” improved on the Subject/Caregiver Global Impression of Change.

The COMT inhibitor helps increase ON time without promoting dyskinesia.

The co-director of the Jane and John Justin Neurosciences Center at Cook Children’s Hospital discussed his experience with Zogenix’s investigational Dravet syndrome treatment in clinical trials and in the Expanded Access Program.

Data from the OVERCOME study of more than 20,000 respondents suggest that those whose acute migraine treatment was optimized according to the mTOQ had less disability and better quality of life.

Ofatumumab, Novartis’s fully human anti-CD20 monoclonal antibody, demonstrated a reduction in the risk of 3- and 6-month confirmed disability progression compared to teriflunomide in relapsing multiple sclerosis.

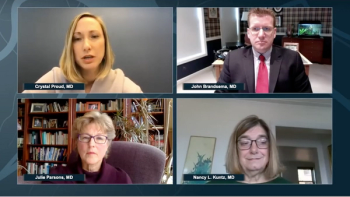
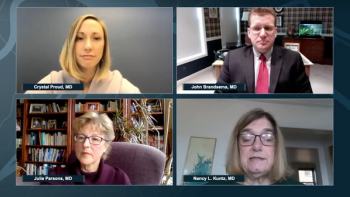
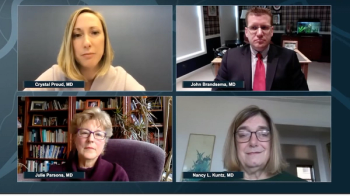
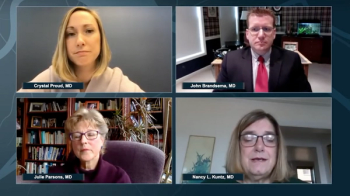
Neurology News Network for the week ending April 25, 2020.

The co-director of the Jane and John Justin Neurosciences Center at Cook Children’s Hospital discussed the real-world data collected from the Expanded Access Program for fenfluramine (Fintepla; Zogenix) in patients with Dravet syndrome.

Take 5 minutes to catch up on NeurologyLive's highlights from the week ending April 24, 2020.