
Patients 65 and under were more likely to develop seizures following a stroke than older patients.

Patients 65 and under were more likely to develop seizures following a stroke than older patients.

The beneficial impact on stroke risk appeared to be greater in patients age 79 and younger than in the older patients examined.

Self-administration of the drug led to statistically significant motor status changes within 30 minutes of treatment.
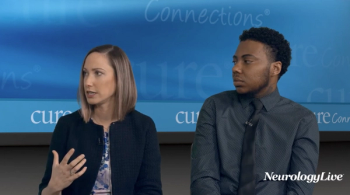

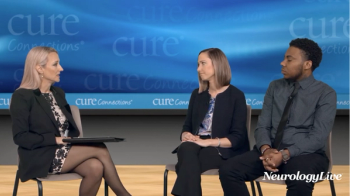
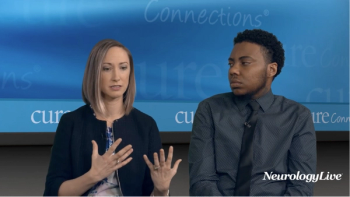
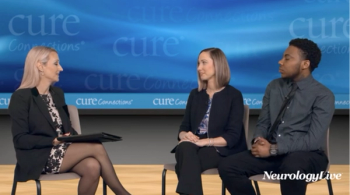
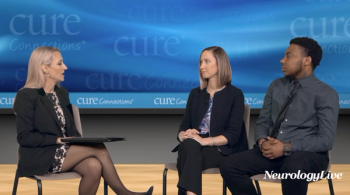
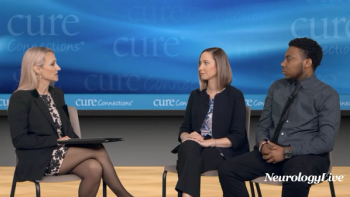
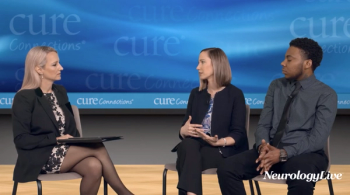
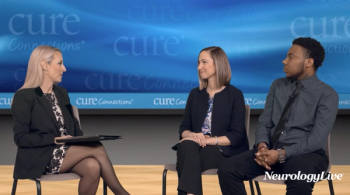

In addition to significant improvements compared to placebo on Quantitative QMG scores, zilucoplan improved patients' activities of daily living and quality of life scores.

The model may help clinicians enact preventative measures for those with migraine with aura at an early stage.

These data confirm the need for consideration of these costs when prescribing treatments, and is of considerable importance as out of pocket costs continue to rise in the US.

The Prometra II offers a pressure-driven, valve-gated delivery mechanism that allows for novel programming modes of intermittent flow followed by periods of no flow—a feature unique to this device.

The announcement formally connects Bcureful and the Tuberous Sclerosis Alliance together after working closely together for over the past 8 years.

The associate professor of neurology and director of the Women With Epilepsy Program at Northwestern University Feinberg School of Medicine discussed the reasons an adult with epilepsy may seek genetic testing and what benefits it can provide.

The chief medical officer of ProMIS Neurosciences comments on how aducanumab will help propel innovation needed to drive the next generation of therapies for Alzheimer disease.

Patients with Parkinson disease with moderate to severe white matter hyperintensity (WMH) burden had a greater risk of developing levodopa-induced dyskinesia compared with those with minimal WMHs.

Good functional recovery at 3 months is achieved in approximately a quarter of patients with acute ischemic stroke with large-vessel occlusion due to calcified emboli.

The chronic migraine preventive, despite falling short of efficacy end points, was safe and well-tolerated in a small cohort of patients aged 12 to 17 years.

The revision accounts for data from the NURTURE trial showing that patients with 3 copies of SMN2 greatly benefit from early treatment.