
Amylyx Pharmaceuticals announced the trial of the 2-drug combination is seeking to enroll 100 patients with late-stage mild cognitive impairment or dementia due to Alzheimer, with plans to complete the study in 2020.

Amylyx Pharmaceuticals announced the trial of the 2-drug combination is seeking to enroll 100 patients with late-stage mild cognitive impairment or dementia due to Alzheimer, with plans to complete the study in 2020.

A new analysis provides a comprehensive update on this significant, and growing, cause of disability and death.

The FDA cited 2 deficiencies in the NDA: certain nonclinical studies were not included to allow for assessment of chronic administration of fenfluramine; and an incorrect version of the clinical data was submitted.

The chief scientific officer at the Parkinson's Foundation spoke about the need to increase awareness about the proper care of patients with Parkinson disease.

The planned phase 1/2 trial of the recombinant AAV5 vector treatment, the first one-time administered AAV gene therapy to enter clinical testing for Huntington disease, is expected to begin dosing patients in the second half of 2019.

The senior preclinical and clinical imaging scientist at the National Institute of Neurological Disorders and Stroke gave a presentation on a volumetric segmented echo-planar-imaging (3D-EPI) sequence, which could be used to detect novel biomarkers such as the central vein sign rapidly.

The International Headache Society has issued a number of recommendations for the proper design of trials for the prevention of pediatric migraine, hoping to address the challenges which are unique to pediatric patient populations.
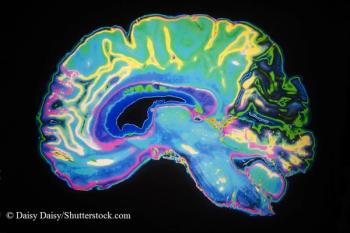
Repeated brain MRI scans show four small gray matter lesions. What’s in your differential?

Data from a national cohort has shown a 48% rate of confirmed symptomatic fractures, which the authors suggested was expected to be a large underestimation of the real frequency in the DMD patient population.

Neurology News Network for the week of April 6, 2019.

The Senior Vice President for Research and Training at Kessler Foundation discussed how cognitive rehabilitation is one approach to addressing cognitive problems in multiple sclerosis.

According to Kyowa Kirin, the investigational selective adenosine A 2A receptor antagonist has had its PDUFA action date set for August 27, 2019.










Here’s a brief look at therapies for neurological disorders that the FDA has approved within the past 6 months.

The director of the Jefferson Headache Center at Jefferson University Hospital discusses the problem with traditional nasal sprays and how improvements in formulation and delivery can improve efficacy.

The Professor in UCLA's Department of Neurology and Director of the UCLA MS Program spoke about disease-modifying therapies that would complement anti-inflammatories by targeting neurodegenerative processes.

Patient management 90 days after amyloid PET changed in 60.2% of patients with MCI and 63.5% of patients with dementia of uncertain etiology.
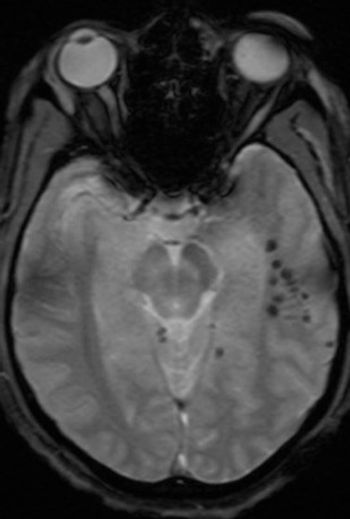
A young man sustained a serious head injury in a car accident, but routine brain CT and MRI scans are normal. Your next step?

The Senior Vice President for Research and Training at Kessler Foundation spoke about research he and colleagues have developed at the Kessler Foundation to aid with cognitive problems in persons with MS.

The administration is seeking help from the medical community and the public to increase risk awareness and report past or future adverse events associated with e-cigarette use.

The professor of neurology at Mayo Clinic spoke about the challenges he faces in diagnosing and treating hereditary ATTR amyloidosis polyneuropathy.
The Professor in UCLA's Department of Neurology and Director of the UCLA MS Program stressed the importance of basing research on clinical observations, understanding them in the lab, then designing novel clincal trials.