
The research grant aims to diversify Friedreich ataxia treatments, addressing challenges in trial design and therapeutic strategies for this condition.

The research grant aims to diversify Friedreich ataxia treatments, addressing challenges in trial design and therapeutic strategies for this condition.

The executive vice president and chief research officer of the Muscular Dystrophy Association shared her reaction to the recent FDA approval of givinostat for Duchenne muscular dystrophy.

Mind Moments®, a podcast from NeurologyLive®, brings you an exclusive interview with Sharon Hesterlee, PhD. [LISTEN TIME: 10 minutes]
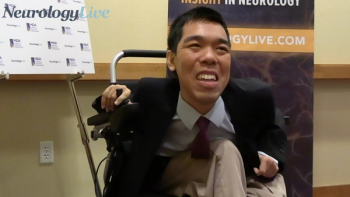
The second-year PhD student in bioinformatics at Boston University who lives with LAMA2 congenital muscular dystrophy talked about the potential impact and challenges of gene therapy in neuromuscular diseases. [WATCH TIME: 5 minutes]

Findings from the recent phase 3b SMART trial affirm the safety and efficacy of intravenous onasemnogene abeparvovec (Zolgensma; Novartis) in spinal muscular atrophy when patient weights range from 8.5 kg to 21 kg.

A recent study presented at MDA 2024 highlighted the evolving respiratory patterns in pediatric patients with Duchenne muscular dystrophy, offering crucial insights for effective respiratory management in this patient population.

A recent analysis of data from the US National Registry presented at MDA 2024 revealed gender disparities in pediatric-onset facioscapulohumeral muscular dystrophy, with girls experiencing more severe outcomes.

A case series presented at the MDA 2024 revealed that ravulizumab infusion intervals show promise in stabilizing symptoms and reducing exacerbations in patients with generalized myasthenia gravis.

A recent study presented at MDA 2024 suggests that preoperative use of disease-modifying agents for patients with spinal muscular atrophy leads to less severe postoperative complications following scoliosis surgery.

In a recent phase 2 trial analysis of viltolarsen presented at MDA 2024, findings showed improvement in forced vital capacity compared with standard care in patients with Duchenne muscular dystrophy.

A new meta analysis of 3 studies presented at MDA 2024 revealed that ataluren significantly slowed the decline in muscle function for patients with nonsense mutation Duchenne muscular dystrophy.

A recent post hoc analysis of the phase 3 EPIDYS trial presented at MDA 2024 revealed significant positive outcomes with givinostat, a histone deacetylase inhibitor, among patients with Duchenne muscular dystrophy.

Median time to loss of ambulation for golodirsen-treated patients was 1968 days vs 1092 days for external control patients.

A new analysis of phase 3 MOVE-FA trial presented at MDA 2024 revealed promising outcomes for vatiquinone, an oral 15-lipoxygenase inhibitor, in patients with Friedreich ataxia.

Over a 52-week treatment period, treatment with SRP-9001 resulted in improvements in secondary outcomes of time to rise, micro-dystrophin expression, and 10-meter walk/run.

Patients with Duchenne muscular dystrophy between ages of 6 and 24 months old demonstrated a similar safety profile on eteplirsen than those between 24 and 48 months of age.

In a real-world study, eterplirsen was safe for patients with Duchenne muscular dystrophy, with sustained or improved status in function.

Tadalafil shows potential in addressing microvascular impairment in Duchenne muscular dystrophy, with post-contractile MRI as a screening tool.

Jeff Chamberlain, PhD, a leading professor in gene therapy focused on Duchenne muscular dystrophy, shared his reaction to being named the recipient of the 2024 MDA Legacy Award, as well as the state of the DMD field currently.

The chief medical advisor at the Muscular Dystrophy Association provided perspective on the upcoming meeting and the conversations surrounding new therapeutics for diseases that once had little to nothing. [WATCH TIME: 4 minutes]

In honor of Rare Disease Day, the vice president of public policy and advocacy at the Muscular Dystrophy Association shared his perspective of advocacy and policy progress in the realm of rare neuromuscular diseases. [WATCH TIME: 6 minutes]

The professor in the Department of Translational Neuroscience at Barrow Neurological Institute provided perspective on the approval of tofersen (Qalsody; Biogen) for patients with ALS and the ways neurofilament light will be used going forward. [WATCH TIME: 3 minutes]

The associate professor of neurology at Columbia University talked about a track session that he will be chairing at the upcoming MDA conference on amyotrophic lateral sclerosis genetic research. [WATCH TIME: 5 minutes]

The professor in the Department of Translational Neuroscience at Barrow Neurological Institute discussed several topics related to ALS research and the emerging biomarkers in recent years ahead of the 2024 MDA Clinical and Scientific Conference.

The professor in the Department of Translational Neuroscience at Barrow Neurological Institute provided comments on her session track at the upcoming 2024 MDA conference and some of the recent advancements in ALS research. [WATCH TIME: 4 minutes]

Ahead of the 2024 MDA Conference, the medical advisor and care center director at Muscular Dystrophy Association talked about the track sessions he will be cochairing at the meeting. [WATCH TIME: 5 minutes]

Ahead of the 2024 MDA Conference, the vice president of public policy and advocacy at the Muscular Dystrophy Association talked about conference advocacy collaboration, access to treatments, and the state of healthcare. [WATCH TIME: 6 minutes]

The director of disability policy at the Muscular Dystrophy Association talked about the future possibility of having an aircraft that accommodates for patients with neuromuscular disorders who use mobility devices. [WATCH TIME: 3 minutes]

The director of disability policy at the Muscular Dystrophy Association discussed his lecture at AANEM 2023 on how clinicians can advocate for their patients to help improve accessibility and health equity. [WATCH TIME: 7 minutes]

The chief medical advisor at the Muscular Dystrophy Association gave his reaction to Amicus Pharmaceuticals’ recently FDA approved 2-part therapy for patients living with Pompe disease. [WATCH TIME: 7 minutes]