APOE ϵ4/ϵ4 Predicts Higher Alzheimer Risk, But Many Without Allele Have True AD Pathology, Study Shows
Key Takeaways
- APOE ϵ4/ϵ4 carriers show higher likelihood of AD-related biomarker results, but non-carriers also exhibit AD pathology, complicating interpretation.
- No significant differences in biomarker results were found between APOE ε3 and ε4 homozygotes, except in p-tau 181 testing.
A new analysis of biomarker testing presented at NSGC 2025 highlighted the complexity of Alzheimer disease risk assessment and underscored the role of genetic counselors in interpreting results.
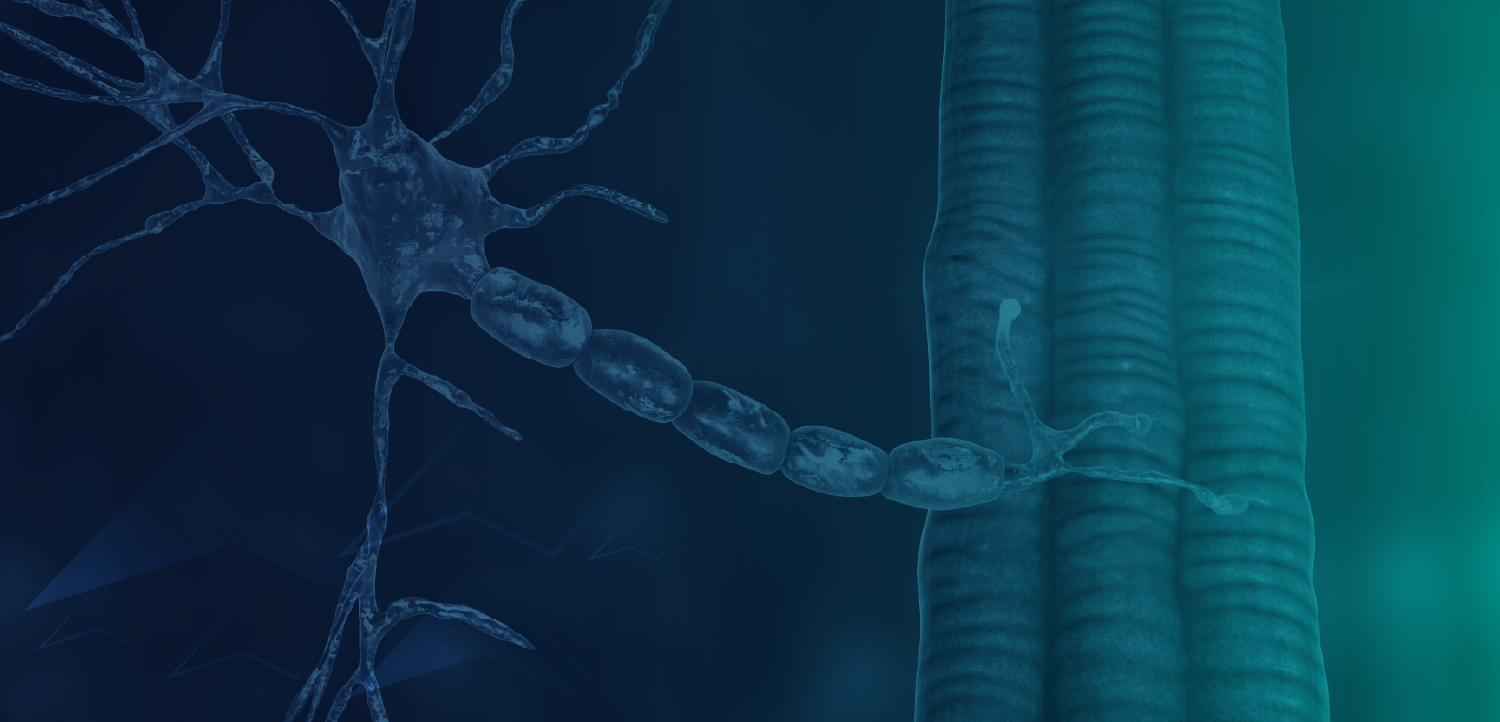
A newly presented exploratory cohort analysis showed that individuals who were apolipoprotein (APOE) ϵ4/ϵ4 carriers were significantly more likely to have biomarker results indicating symptomatic or at-risk status for mild cognitive impairment or Alzheimer disease (AD)–associated dementia.1 However, a substantial number of individuals without the APOE ϵ4 allele also showed biomarker evidence of AD pathology, highlighting the complexity of interpreting biomarker results.
The study was presented by lead author Matt Tschirgi, MS, CGC, senior manager of genomic services at Quest Diagnostics, at the
In this study, researchers retrospectively reviewed consecutive results from adults in the United States and its territories who underwent the most recent peripheral blood APOE allele testing at a CLIA-certified diagnostic laboratory for AD. Authors noted that a subset of participants also received blood biomarker testing. Investigators performed APOE genotyping using restriction endonuclease digestion of PCR-amplified genomic DNA, and biomarker levels were reported as previously described.4
READ MORE:
Among 7313 individuals who underwent p-tau 181 testing, researchers reported that 49.5% of them had results consistent with mild cognitive impairment (MCI) or AD-related dementia. Researchers also observed that significantly more participants homozygous for APOE ε4 had biomarker results consistent with MCI or dementia compared with those without the ε4 allele (67.8% vs 40.5%; P <.0001).1
Of 743 individuals who received p-tau 217 testing, findings revealed that 56.0% had results consistent with MCI or dementia because of AD. Notably, this proportion of individuals did not significantly differ between participants homozygous for APOE ε3 and those homozygous for APOE ε4. Authors concluded that certified genetic counselors may possess the expertise to interpret discordant or ambiguous results to support informed decision-making, a role that may become increasingly important as additional AD therapies become available.
The growing attention on AD because of emerging therapies, biomarker testing, and recent publications has prompted discussion regarding the role of genetic counselors in testing.5 According to the study authors, current discussions on NSGC electronic mailing lists about testing practices have generated multiple questions with few definitive answers. Although AD is genetically heterogeneous, APOE allele status remains the strongest known genetic risk factor for late-onset disease. Despite APOE testing being available for many years, researchers noted that newer blood biomarker assays may add complexity to counseling discussions regarding dementia and testing.
REFERENCE
1. Powis Z, Tschirgi M, Liaquat K, Racke M. A Tau-tally New Era: Genetic Counseling Meets Alzheimer's Biomarker Testing. Presented at: 2025 NSGC Annual Conference; November 6-10; Seattle, Washington.
2. Fortea J, Pegueroles J, Alcolea D, et al. APOE4 homozygozity represents a distinct genetic form of Alzheimer's disease. Nat Med. 2024;30(5):1284-1291. doi:10.1038/s41591-024-02931-w
3. Weber DM, Stroh MA, Taylor SW, et al. Development and Clinical Validation of Blood-Based Multibiomarker Models for the Evaluation of Brain Amyloid Pathology. Neurol Clin Pract. 2025;15(6):e200546. doi:10.1212/CPJ.0000000000200546
4. Jack CR Jr, Andrews JS, Beach TG, et al. Revised criteria for diagnosis and staging of Alzheimer's disease: Alzheimer's Association Workgroup. Alzheimers Dement. 2024;20(8):5143-5169. doi:10.1002/alz.13859
5. Weber DM, Stroh MA, Taylor SW, et al. Development and Clinical Validation of Blood-Based Multibiomarker Models for the Evaluation of Brain Amyloid Pathology. Neurol Clin Pract. 2025;15(6):e200546. doi:10.1212/CPJ.0000000000200546
Newsletter
Keep your finger on the pulse of neurology—subscribe to NeurologyLive for expert interviews, new data, and breakthrough treatment updates.