
Epilepsy
Latest News

Latest Videos

CME Content
More News

The director of the adult epilepsy center at Washington University in St. Louis spoke about the use of diazepam nasal spray in patients with epilepsy ­and detailed the advantages it offers these patients and their physicians.

The professor of medicine, neurology, at the University of Toronto spoke to the hurdles faced by both pediatric and adult neurologists when transitioning a patient with epilepsy from childhood care to adult care.

A boy diagnosed with glycine encephalopathy in the newborn period was initiated on the ketogenic diet at 11 years-old for the treatment of medication refractory epilepsy.
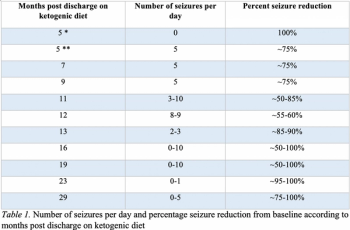
A boy diagnosed with glycine encephalopathy in the newborn period was initiated on the ketogenic diet at 11 years-old for the treatment of medication refractory epilepsy.

Targeting research in cognitive impairment in epilepsy primarily on seizures themselves suggests that a patient’s comorbid problems will resolve with seizure control, though that is not always the case.

The director of comprehensive epilepsy center and professor of neurology at Thomas Jefferson University spoke to the evolution of epilepsy interventions over the last decade and its effect on the level of care for patients.

Rodney Allan Radtke, MD, professor of neurology at Duke University School of Medicine in Durham, North Carolina, discussed some of the new therapeutic options available to help improve tolerance and adherence challenges that clinicians commonly face in epilepsy practice.

The professor of neurology at the Icahn School of Medicine at Mount Sinai discussed what still needs to be done to clarify medical marijuana’s position in medicine.

A 23-year-old male college student presents with first generalized tonic-clonic seizure followed by episodes of violent psychosis.

The associate chief of the MS division and professor of neurology at the University of Pennsylvania described the relationship between artificial intelligence and medicine, and how he sees it evolving in the future.

Neurology News Network for the week ending August 17, 2019.
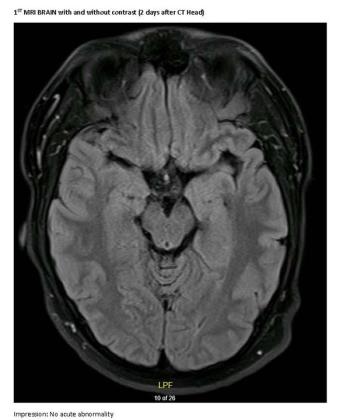
A generally healthy, young adult male presents with first generalized tonic-clonic seizure followed by episodes of violent psychosis.

The pediatric neurologist and epilepsy specialist at Children’s Hospital Colorado spoke about patient interest in cannabidiol after the FDA approval of Epidiolex, and what remains to be done to perfect its use in epilepsy.

The product includes cannabidiol that is encased in a gelatin bead inside a gastro-resistant capsule to help promote optimal absorption and reduce adverse events associated with other oral CBD formulations.

The new drug application for Libervant is expected to be completed in the fourth quarter of 2019, which if approved, would offer a potentially first in class oral treatment for breakthrough or cluster seizures.

Neurology News Network for the week ending August 10, 2019.

Exposure to the care of either a neurologist or comprehensive epilepsy program epileptologist resulted in a significantly lower rate of premature mortality than that of those who were not, based on findings from a cohort of more than 20,000 cases.

The associate professor of neurology and medical director of the epilepsy monitoring unit and Penn epilepsy surgical program at the University of Pennsylvania discussed the findings of a single-center review of off-label clobazam use for patients with drug-refractory epilepsy.

The new drug application for Libervant is expected to be completed in the fourth quarter of 2019, which if approved, would offer a potentially first in class oral treatment for breakthrough or cluster seizures.

The associate professor of neurology and medical director of the epilepsy monitoring unit and Penn epilepsy surgical program at the University of Pennsylvania shared insight into the prioritization of women with epilepsy who may intend to get pregnant.

The clinical neuropsychologist and head of the School of Psychological Sciences at the University of Melbourne discussed the results of a study she and colleagues conducted in which they mapped the long-term social outcomes of patient post-epilepsy surgery.

The clinical neuropsychologist and head of the School of Psychological Sciences at the University of Melbourne detailed how the transition from states of illness to wellness after epilepsy surgery can result not just in brain changes, but psychosocial challenges for patients.

Mind Moments® a podcast from NeurologyLive®, brings you exclusive interviews with experts in neurologic disorders.

The staff neurologist at Cleveland Clinic’s Mellen Center for MS shared her insight into the use of telemedicine in an outpatient setting across a number of subspecialties in neurology and how it can supplement care going forward.

The clinical neuropsychologist and head of the School of Psychological Sciences at the University of Melbourne spoke about what physicians need to provide to help patients through the adjustment period after epilepsy surgery.

















