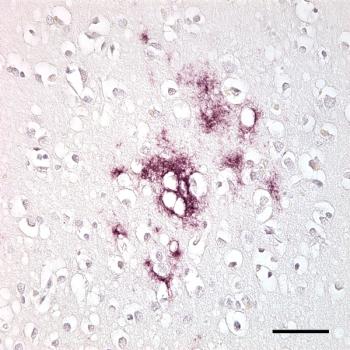
One year post-launch, the Ambry Genetics and Akcea Therapeutics hereditary ATTR amyloidosis testing program has been used by more than 700 physicians. The free test screens for up to 81 genes that cause hereditary polyneuropathies and up to 92 genes associated with hereditary cardiomyopathies, including hATTR amyloidosis.