Neuromuscular
Latest News
CME Content

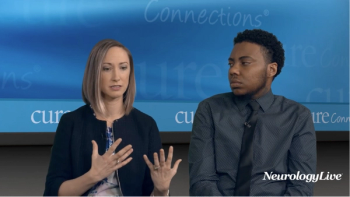
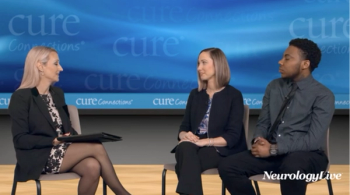
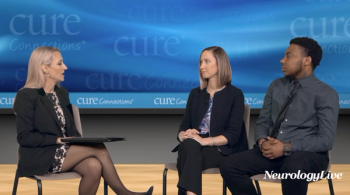

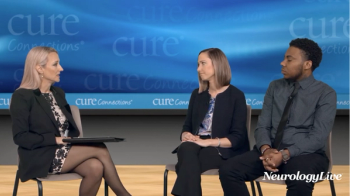
In addition to significant improvements compared to placebo on Quantitative QMG scores, zilucoplan improved patients' activities of daily living and quality of life scores.

The Prometra II offers a pressure-driven, valve-gated delivery mechanism that allows for novel programming modes of intermittent flow followed by periods of no flow—a feature unique to this device.

The announcement formally connects Bcureful and the Tuberous Sclerosis Alliance together after working closely together for over the past 8 years.

The revision accounts for data from the NURTURE trial showing that patients with 3 copies of SMN2 greatly benefit from early treatment.

The Tuberous Sclerosis Alliance has announced updates to access of everolimus, a drug used to treat several conditions associated with tuberous sclerosis complex.

Neurofilament light chain has emerged as a biomarker with utility across the breadth of neurology, but just how much can it actually help?

The Nippon Shinyaku investigational DMD agent has a Prescription Drug User Fee Act date within the third quarter of 2020.

New findings suggest a strong association between certain lower extremity biomarkers and measures of clinical function in patients with Duchenne muscular dystrophy.

The biotech company plans to roll out 2 clinical trials for patients with amyotrophic lateral sclerosis following the orphan drug designation for PrimeC.

Neurology News Network for the week ending February 8, 2020.

The study is the first placebo-controlled trial to include non-ambulant adults with spinal muscular atrophy type 2 or 3.









