
Catch up on any of the neurology news headlines you may have missed over the course of October 2024, compiled all into one place by the NeurologyLive® team.

Catch up on any of the neurology news headlines you may have missed over the course of October 2024, compiled all into one place by the NeurologyLive® team.

Test your neurology knowledge with NeurologyLive®'s weekly quiz series, featuring questions on a variety of clinical and historical neurology topics. This week's topic is on PIRA (progression independent of relapse activity) in multiple sclerosis.

The double-blind, placebo-controlled trial will include 200 patients with Angelman syndrome who will be followed over a 12-month period, with an additional open-label extension after.

David Chernoff, MD, chief medical officer at SetPoint Medical, provided clinical insight on a new pilot study intended to assess the therapeutic potential of a rechargeable neurostimulation aimed to reducing demyelination in relapsing multiple sclerosis.

Neurology News Network. for the week ending November 9, 2024. [WATCH TIME: 4 minutes]

Despite not meeting its primary end point, treatment with GV1001 showed more promising results in patients with progressive supranuclear palsy-Richardson’s syndrome over a 24-week treatment period.

Take 5 minutes to catch up on NeurologyLive®'s highlights from the week ending November 8, 2024.
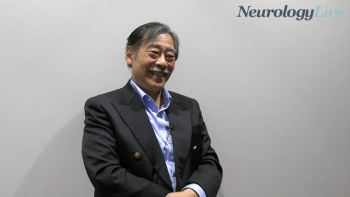
The professor of neuropathology at the University of Tokyo School of Medicine talked about the latest data on lecanemab use in clinical practice for Alzheimer disease in Japan presented at CTAD 2024. [WATCH TIME: 4 minutes]

M. Scott Perry, MD, an expert in epileptic disorders and board president of PERC, provided insights on the group's mission and goals, its structure and membership, and its 60+ ongoing research projects.

Nina Miolane, PhD, and Amy Kuceyeski, PhD, the codirectors of the Ann S. Bowers Women's Brain Health Initiative AI Core, provided their commentary on the challenges and progress made in women’s neurology.

In previously reported data, AOC 1020 demonstrated a consistent reduction in double homeobox 4 regulated genes among patients with facioscapulohumeral muscular dystrophy at 4 months.

The head of global clinical development for immunology/bone at UCB talked about findings presented at CTAD 2024 from the phase 2 trial assessing bepranemab in Alzheimer disease. [WATCH TIME: 3 minutes]

Rapport Therapeutics’ is actively recruiting patients for its phase 2a trial investigating its lead therapy candidate RAP-219 among patients with focal epilepsy, with topline data anticipated in mid-2025.

The vice president of scientific engagement at the Alzheimer's Association talked about the emergence of blood biomarker tests as valuable diagnostic tools for Alzheimer disease in the clinical setting. [WATCH TIME: 5 minutes]

The cAPPricorn-1 Phase 2 trial will assess mivelsiran’s efficacy in CAA, focusing on reducing the annualized rate of new cerebral microbleeds over 24 months.

Diego Torres-Russotto, MD, chair of neurology at Baptist Health Miami Neuroscience Institute, discussed the Miami Neuroscience Symposium’s focus on addressing the community’s neurological needs, balancing content delivery and fostering multidisciplinary collaboration.

Michael McDermott, MD, chief medical executive at Baptist Health Miami Neuroscience Institute, gave greater insights on the institute’s annual symposium, which offers attendees updates in cutting-edge imaging, surgical techniques, and neurocognitive research.

The professor of psychiatry and pharmacology at the University of Toronto talked about results from a post hoc analysis presented at CTAD 2024 that explored synthetic cannabinoid nabilone for agitation in Alzheimer disease. [WATCH TIME: 4 minutes]

Michael McDermott, MD, chief medical executive at Baptist Health Miami Neuroscience Institute, gave greater insights on the institute’s annual symposium, which offers attendees updates in cutting-edge imaging, surgical techniques, and neurocognitive research.

A trio of experts from Sinaptica Therapeutics talked about recent findings presented at CTAD 2024 from a study assessing personalized transcranial magnetic stimulation in patients with Alzheimer disease. [WATCH TIME: 5 minutes]

The director of the myasthenia gravis clinic at Yale University discussed the therapeutic potential of inebilizumab, an FDA-approved treatment for NMOSD, in myasthenia gravis, based on data from the phase 3 MINT study.

The NDA includes data from a global placebo-controlled, 72-week study as well as findings from the STRIDE registry, an ongoing, observational, real-world study of ataluren in routine care.

STS101 showed promising results in previous trials, with significant pain relief and minimal adverse events reported, reinforcing its potential as a viable treatment for patients with migraine.
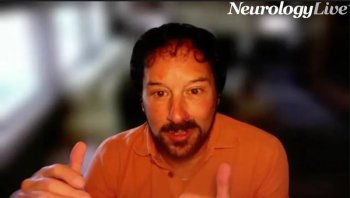
The director of the Muscular Dystrophy Clinic at UCSF Benioff Children’s Hospital provided insight on promising data from the phase 2/3 VIBRANCE-MG study assessing investigational nipocalimab in adolesents with myasthenia gravis. [WATCH TIME: 3 minutes]

Using a Brain-Chip model, investigators demonstrated that pepinemab can inhibit or reverse damage from toxic alpha-synuclein aggregates, with similar results for amyloid-ß expected soon.

A feature on NeurologyLive®, IJMSC Insights offers a closer look at the latest research and the people behind it from the community of the International Journal of Multiple Sclerosis Care (IJMSC) and the Consortium of Multiple Sclerosis Centers (CMSC).

Clinicians discussed the complexities of diagnosing Long COVID, emphasizing the need for improved biomarkers and diagnostic technologies to better serve affected patients.

Over a 48-week treatment period, once daily blarcamesine slowed clinical decline in patients with early-stage Alzheimer disease, with even more pronounced effects in pre-specified common SIGMAR1 wild type group.

The multidisciplinary, 1-day scientific forum will be held May 15, 2025, in Charleston, South Carolina, focusing on nervous system health and care, and reducing neurological inequities and disparities.

Nominations are now being accepted until January 31, 2025. Nominations should be submitted to [email protected].