
Future analysis evaluating the optimal candidate for disease and duration of treatment for the use of tofacitinib in autoimmune encephalitis is needed, according to the study authors.

Future analysis evaluating the optimal candidate for disease and duration of treatment for the use of tofacitinib in autoimmune encephalitis is needed, according to the study authors.

The director of the Lou Ruvo Center for Brain Health and neurologist at Cleveland Clinic discussed the research opportunities for repurposed anti-inflammatory drugs for Alzheimer disease.

The assistant professor of neurology at Mayo Clinic detailed the areas of autoimmune encephalitis research that need more attention, as well as the diagnostic potential of autoantibody assays.

Garey H. Noritz, MD, walks us through a virtual pediatric neurological exam and notes examination findings that may be identified in an infant with Spinal Muscular Atrophy (SMA).
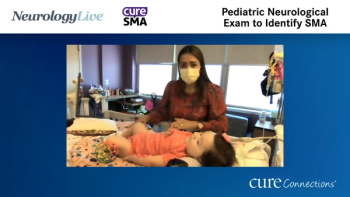
Diana Castro, MD walks us through a pediatric neurological exam and notes examination findings that may be identified in an infant with Spinal Muscular Atrophy (SMA).
The neurologist from the University of Washington Medical Center discussed how the study of natalizumab adverse events came about and the importance of this monitoring period.

Researchers found that GCIPL thickness, along with disease duration of at least 9 years at baseline, were associated with the highest odds of EDSS worsening.

The retrospective analysis in patients aged 55 years and older underlines the importance of testing different patient populations.

The PhD candidate at the neuroimmunology laboratory at Memorial University of Newfoundland discussed further research she would like to see conducted with IL-1RA in MS.

Treatment with masitinib was shown to prevent the amyloid-induced hemichannel-dependent mast cell activity in bone marrow-derived mast cells and brain mast cells.

The director of research analytics at Cure SMA detailed the ways to decrease care management costs for patients with spinal muscular atrophy and the role incoming treatments can play.

The director of the Institute for Health, Health Care Policy and Aging Research at Rutgers University discussed a recently published report on meeting the challenge of caring for persons with dementia and their care partners and caregivers.

Researchers found that sleep disorder-related symptoms were positively correlated with polysomnography indices.

The chair of the Department of Neurology and the director of the Neuroscience Research Institute at The Ohio State University discussed the takeaways from his lecture at ACTRIMS Forum 2021.

A systematic review of 43 studies suggests that MR-guided LITT and radiofrequency ablation had lower rates of Engel Class I outcomes, but fewer major complications post-procedure.

The disposable NeuroCap allows for minimal chances of flawed EEG due to all of the contact points between scalp and electrode sensors being pre-gelled.
The chief scientific officer of the Parkinson’s Foundation also talked about the recent PD Health @ Home series created by the foundation.

Researchers found that, in an exploratory analysis of 150 patients, African American patients with MS were most likely to become disabled and lose employment.

In a study of more than 600 patients, single medication classes were overused by 70% of patients, and less than half of the cohort was taking a preventive treatment.

The chair of the Department of Neurology and the director of the Neuroscience Research Institute at The Ohio State University also offered his insight on discontinuing treatment in progressive MS.

Ishu Arpan, PhD, senior research associate at Oregon Health & Science University, discussed her team’s investigations into identifying patients with MS at risk of falling.

Increases in reperfusion treatment were the largest in lowest-volume hospitals, among rural residents, and among patients aged 85 years and older.
The director of the Lou Ruvo Center for Brain Health and neurologist at Cleveland Clinic provided an overview on how lenalidomide, an FDA-approved cancer drug, will be evaluated in Alzheimer disease.

Here's what is coming soon to NeurologyLive.

The 2-stage study is an open-label, 2-stage clinical trial designed to evaluate safety and dose-escalation (stage 1) and safety and efficacy (stage 2) of surgically delivered AXO-AAV-GM2.

The duo from Montefiore Medical Center discussed new modifications to the 2017 International League Against Epilepsy (ILAE) classification of seizures and epilepsies, relevant to neonates.

Patients having a relapse, confirmed disability accumulation, or worsening in fatigue had significant negative impact on each of the work productivity and activity impairment measures.

The neurologist from Massachusetts General Hospital discussed physician rationales behind prescribing DMT use in RIS.

Researchers found that the percentage of telemedicine visits increased from 15% to 72.8% during the COVID-19 pandemic.

Researchers found that higher NIHSS scores on admission and a successful first-pass effect were predictors of reaching early neurological improvement.