
Researchers saw the least amount of adverse events and the least time to stable dose in participants entering the study with SXB treatment.

Researchers saw the least amount of adverse events and the least time to stable dose in participants entering the study with SXB treatment.

Researchers from the University of Utah analyzed the use of their telestroke system with 27 outside “stroke” facilities.
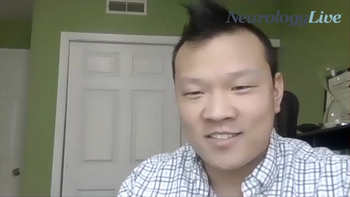
The neurologist at University of Utah discussed his research presented at AAN 2021 on telestroke’s accuracy of differentiating between acute ischemic stroke and mimics.

Patients in the randomized controlled trial and the open-label extension treated with inebilizumab experienced similar rates of being attack-free, extending as far as 4 years.

The professor of neurology and Neurological Sciences, Pediatrics, and Genetics at Stanford Medicine discussed the ULTIMATE trial data presented at the 2021 AAN Annual Meeting.

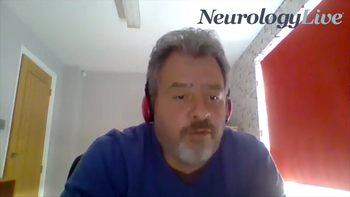
The professor of neurology at Mayo Clinic discussed the need for understanding more about how social determinants of health can impact epilepsy treatment delays.
The dean at the University of Exeter Medical School discussed the questions related to treating dementia-related psychosis and the options available.

Patient/guardian decision, adverse event, and physician decision were the 3 noted reasons for discontinuation with either ofatumumab or teriflunomide.

In an AAN plenary talk, Mark H. Tuszynski, MD, PhD, detailed the work he and colleagues have done to push stem cell therapy from the lab to the clinic to improve care for spinal cord injury.

Researchers analyzed disparities in stroke rehab and found that women and African Americans had the lowest functional performance.

The chief of Movement Disorders Division at the University of Miami Miller School of Medicine discussed some of the recent focused efforts to uncover more about Parkinson disease and what is on the horizon.

Patients with a previous history of cardiovascular risks experienced similarly low number of cardiovascular events with treatment of fremanezumab compared with placebo.

In total, 50% of patients eligible for device-aided therapies did not report having any discussion with providers about future device-aided therapies.

The 2021 AAN Meeting Hot Topics plenary focused on neurological facets of COVID-19, and featured insight from Anthony Fauci, MD, of NIAID, and Walter Koroshetz, MD, of NINDS, among others.

The vascular neurology fellow at the University of Maryland Medical Center discussed strategies to improve stroke care efficiency in women and African Americans.

An AAN session included presentations of research on the latest advancements and knowledge in neuromodulation for patients with these disorders.

The associate professor and director of the Headache Medicine Fellowship Program at Thomas Jefferson University discussed 2 research projects she led presented at AAN 2021 on the efficacy and safety of fremanezumab.

Here's what is coming soon to NeurologyLive.
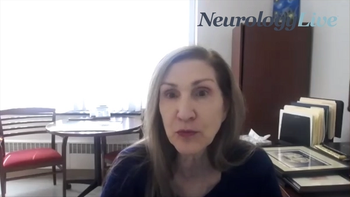
The chief of neurology at Ascension Saint Agnes discussed how providing an overview of patient-specific risk factors and mitigation can improve poststroke care.

Aducanumab currently sits before the FDA for review for the treatment of Alzheimer disease, with a PDUFA date set for June 7, 2021.

Results from the ongoing clinical trial will help define clinical management guidelines for switching patients with relapsing MS on other disease-modifying therapies to siponimod.

No stimulation adverse effects were reported, and patients on the MICC stimulation alone resulted in greater than 50% improvement in UPDRS motor scores.

Researchers from Ascension Saint Agnes improved compliance in their hospital in providing patient-specific, stroke-dedicated discharge instructions.

The vascular neurology fellow at the University of Maryland Medical Center discussed findings that African American women had the lowest functional performance in stroke rehabilitation.

Serious adverse events occurred in 3.7% and 2.4% of subjects in the subgroups of patients with 1 and 2 or more triptan failures, respectively.

The professor of neurology at Mayo Clinic discussed the reasoning for the research he presented at AAN 2021 on social determinants and their impact on epilepsy treatment times.

Neurology News Network for the week ending April 17, 2021.

The study presented at the 2021 AAN Meeting follows the complete response letter issued by the FDA to Acadia Pharmaceuticals due to deficiencies in their sNDA.

Take 5 minutes to catch up on NeurologyLive's highlights from the week ending April 16, 2021.

The chief of neurology at Ascension Saint Agnes discussed her team’s efforts to increase compliance in providing patient-specific stroke DCI.